


have zero kinetic velocity and simultaneously be. Similarly, the gas pressure is higher if the average velocity of molecules is higher. For example, enter 10 to see the effect of a 10 increase above the standard temperature at the current altitude. He proposed that zero on his temperature scale (waters boiling point) would be calibrated at the mean. As the number of molecules increases, the number of collisions and thus the pressure increase. These collisions are the source of pressure in a gas. Because a huge number of molecules will collide with the wall in a short time, we observe an average force per unit area. 3) Algebraically rearrange the Arrhenius equation to get T at k, and use that formula in Excel to get the mean kinetic temperature. In this tutorial we are going to use a land cover classification for the definition of surface emissivity, which is required for the calculation of the land. The MKT is widely used in the pharmaceutical industry.
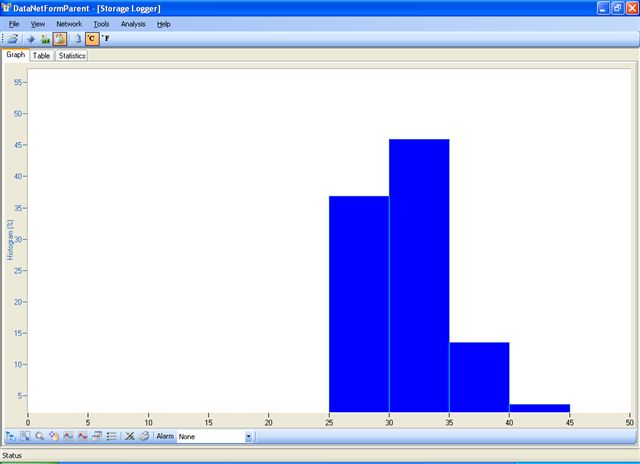
Mean kinetic temperature (MKT) is a simplified way of expressing the overall effect of temperature fluctuations during storage or transit of perishable goods. Engineers and Students worldwide, Our website features more than few hundred calculators for solving complex.
MEAN KINETIC TEMPERATURE CALCULATOR FREE
2) use the AVERAGE () function on this column to get the average k. Calculation giving the natural logarithm of the temperature number. TRANSLATE: Welcome to CALCULATOR EDGE, an online FREE Engineering Calculators. Details: 1) calculate k at each temperature in an adjacent column. A force is thus exerted on the wall, creating pressure.įigure 1 shows an elastic collision of a gas molecule with the wall of a container, so that it exerts a force on the wall (by Newton’s third law). Mean Kinetic Temperature - Excel Help Forum. Calculating Kinetic Energy and Speed of a Gas Molecule (a) What is the. When a molecule collides with a rigid wall, the component of its momentum perpendicular to the wall is reversed. The root-mean-square (rms) speed of a molecule, or the square root of the average.


 0 kommentar(er)
0 kommentar(er)
